Advances in CPB Circuit Coatings: Impact on Biocompatibility and Inflammatory Response
- Home
- Cannulation
- Current Page
Introduction
Cardiopulmonary bypass (CPB) is a lifesaving technology in cardiac surgery, but it introduces blood-material interactions that trigger inflammatory and coagulative responses. To address these issues, advancements in biocompatible CPB circuit coatings have been developed to reduce platelet activation, complement system activation, and systemic inflammatory response syndrome (SIRS). This article explores the latest innovations in CPB circuit coatings and their impact on biocompatibility and inflammation.
The Biocompatibility Challenge in CPB
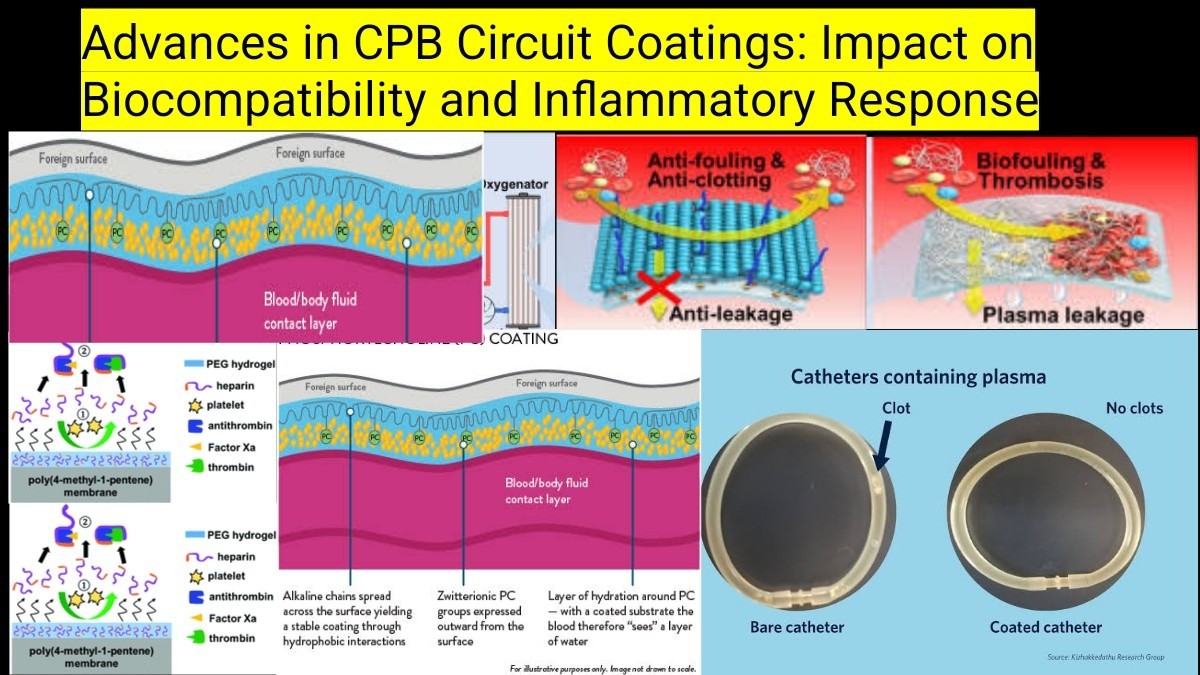
Traditional CPB circuits are made of polyvinyl chloride (PVC) and polycarbonate, which induce protein adsorption, platelet activation, and leukocyte adhesion, leading to an inflammatory cascade (Larmann & Theilmeier, 2016). The body’s response to foreign surfaces includes:
- Activation of coagulation pathways, leading to thrombus formation.
- Inflammatory cytokine release, increasing vascular permeability.
- Oxidative stress, contributing to endothelial dysfunction.
Advances in biocompatible coatings aim to mitigate these adverse effects by modifying surface properties and reducing blood activation.
Types of CPB Circuit Coatings
1. Heparin-Based Coatings
Mechanism: Heparin-coated circuits provide an antithrombotic surface by binding antithrombin III (AT-III), inhibiting thrombin formation (Ranucci et al., 2019).
Benefits:
- Reduces platelet consumption and fibrin deposition.
- Lowers systemic anticoagulation requirements.
- Decreases post-operative bleeding risks.
Limitations:
- Heparin can be neutralized by protamine, reducing efficacy.
- Patients with heparin-induced thrombocytopenia (HIT) require alternative coatings.
2. Phosphorylcholine (PC)-Based Coatings
Mechanism: PC mimics natural cell membranes, reducing protein adsorption and platelet adhesion (Gorbet & Sefton, 2004).
Benefits:
- Enhances blood compatibilitywithout affecting coagulation.
- Reducescomplement system activation and inflammation.
Limitations:
- Less effective in prolonged CPB durations compared to heparin.
3. Albumin and Polyethylene Oxide (PEO) Coatings
Mechanism: Albumin-based coatings prevent protein adsorption, while PEO provides a hydrated surface, reducing cellular interactions (Bevan et al., 2020).
Benefits:
- Minimizesplatelet and leukocyte adhesion.
- Reduces pro-inflammatory mediator release.
Limitations:
- Limited durability inlong-duration CPB procedures.
4. Nitric Oxide (NO)-Releasing Coatings
Mechanism: NO coatings mimic endothelial function, inhibiting platelet aggregation and adhesion (Seah et al., 2022).
Benefits:
- Strong antithrombotic and anti-inflammatory properties.
- Preventsmicrovascular dysfunction post-CPB.
Limitations:
- Still in early clinical trials, requiring further validation.
Impact on Inflammatory Response
Biocompatible coatings significantly reduce CPB-induced inflammation by minimizing cytokine release, leukocyte activation, and complement activation. Studies show:
- Heparin-coated circuits lower interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels (Ranucci et al., 2019).
- Phosphorylcholine coatings reduce platelet consumption and fibrinolysis markers (Gorbet & Sefton, 2004).
- NO-releasing coatings prevent endothelial dysfunction, improving postoperative organ function (Seah et al., 2022).
Future Perspectives
Advancements in CPB circuit coatings continue to evolve, with research exploring:
- Nanotechnology-based coatings for enhanced thromboresistance.
- Hybrid coatings combining heparin, phosphorylcholine, and NO donors.
- Personalized CPB circuits, tailored to patient-specific inflammatory profiles.
Conclusion
Innovations in CPB circuit coatings have significantly improved biocompatibility and inflammatory control, leading to better patient outcomes. While heparin-based coatings remain the gold standard, newer phosphorylcholine, albumin, and NO-releasing coatings are promising alternatives. Ongoing research will further refine biocompatibility strategies to reduce CPB-related complications and enhance cardiac surgery outcomes.
References
- Bevan, R., et al. (2020). «Advances in blood-compatible coatings for extracorporeal circuits.» Journal of Biomaterials Science, 31(10), 1257-1273.
- Gorbet, M. B., & Sefton, M. V. (2004). «Biocompatibility score for evaluation of blood-contacting biomaterials.» Journal of Biomedical Materials Research, 69A(2), 221-230.
- Larmann, J., & Theilmeier, G. (2016). «Inflammatory response to cardiac surgery: Cardiopulmonary bypass vs. off-pump surgery.» Best Practice & Research Clinical Anaesthesiology, 30(1), 15-30.
- Ranucci, M., et al. (2019). «Heparin-coated circuits and inflammatory response in CPB: A meta-analysis.» The Annals of Thoracic Surgery, 108(5), 1485-1492.
- Seah, J., et al. (2022). «Nitric oxide-releasing coatings for cardiopulmonary bypass circuits: A breakthrough in biocompatibility.» American Journal of Physiology – Heart and Circulatory Physiology, 322(4), H560-H570.
Asif Mushtaq: Chief Perfusionist at Punjab Institute of Cardiology, Lahore, with 27 years of experience. Passionate about ECMO, perfusion education, and advancing perfusion science internationally.
