The Air-Blood Interface During Cardiopulmonary Bypass: Mechanisms and Clinical Implications
- Home
- Cannulation
- Current Page
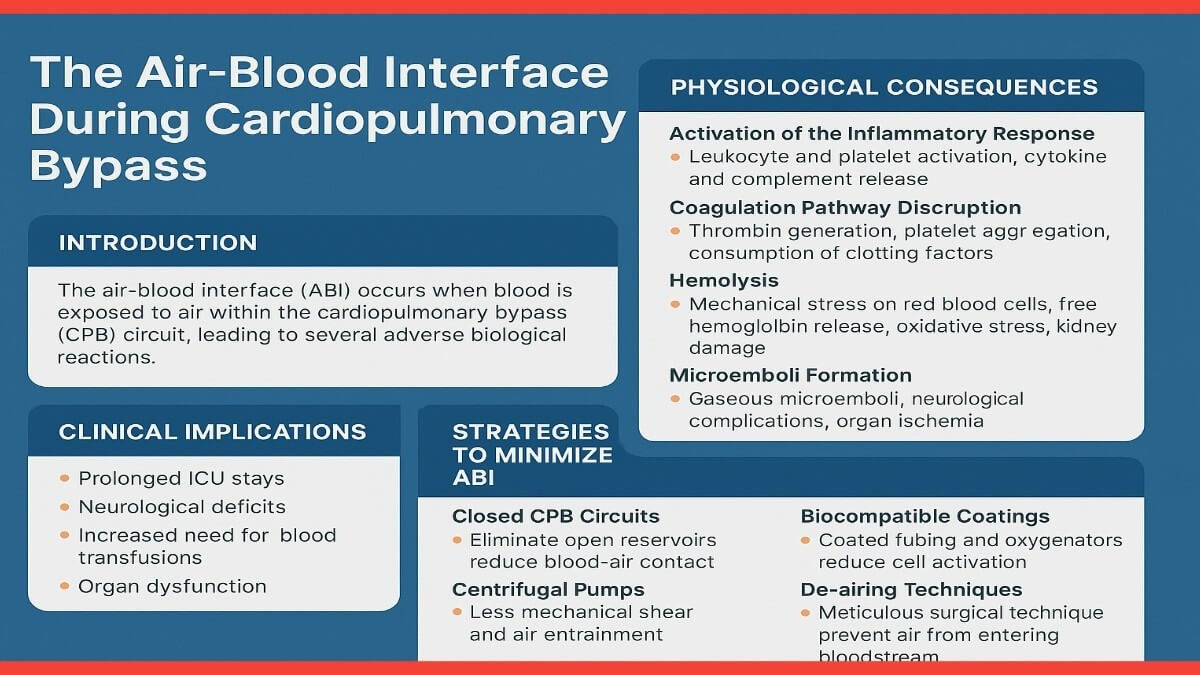
Introduction: Cardiopulmonary bypass, a cornerstone of modern cardiac surgery, temporarily diverts blood away from the heart and lungs, allowing for surgical intervention in a controlled environment. During CPB, blood is circulated through an extracorporeal circuit where it is oxygenated and then returned to the patient. However, this process differs from normal physiology in several respects, one of the most significant being the direct exposure of blood to air. Unlike the closed circulatory system, the CPB circuit often includes open reservoirs and aspirated suction lines that introduce air into the system. This air-blood interface is implicated in initiating a systemic inflammatory cascade and other adverse effects that complicate patient recovery (Toomasian et al., 2018).
Pathophysiological Mechanisms:
- Inflammatory Activation: The air-blood interface stimulates an acute inflammatory response by activating leukocytes and platelets. The surface contact with air induces degranulation of polymorphonuclear leukocytes and promotes the release of inflammatory cytokines, including IL-6, IL-8, and TNF-α (Carr et al., 2020). These cytokines increase endothelial permeability and contribute to vascular leakage, pulmonary edema, and myocardial depression. Furthermore, the complement system is activated, particularly via the alternative and lectin pathways, amplifying the inflammatory response (Paparella et al., 2002).
- Hemolysis: The mechanical trauma induced by roller and centrifugal pumps, combined with ABI, contributes to red blood cell lysis. The resulting free hemoglobin can scavenge nitric oxide, impairing vasodilation and contributing to vasoconstriction and hypertension. Additionally, free hemoglobin is filtered by the kidneys, where it can precipitate tubular necrosis, exacerbating the risk of acute kidney injury (AKI) (Paparella et al., 2002).
- Coagulation and Hemostatic Disruption: Air exposure causes activation of the intrinsic pathway of coagulation through contact with negatively charged surfaces and air-liquid interfaces. Platelet adhesion and aggregation are promoted, leading to microthrombus formation. This consumptive coagulopathy may coexist with increased fibrinolysis, resulting in coagulopathic bleeding during and after surgery. Studies have shown increased D-dimer levels and reduced platelet count associated with extended air exposure during CPB (Allen et al., 2005).
- Gaseous and Particulate Microemboli Formation: ABI facilitates the formation of microemboli, which may be air, lipid, or fibrin-based. These emboli can bypass the pulmonary filter during CPB, especially in the presence of intracardiac shunts or arterial air entry. Neurological outcomes such as postoperative delirium, stroke, and long-term cognitive deficits have been linked to microembolic load during cardiac surgery (Bellinger et al., 2001).
Clinical Implications: Patients undergoing CPB are at elevated risk for complications directly related to ABI. SIRS is frequently observed, with systemic cytokine elevation leading to multi-organ involvement. Pulmonary complications such as acute lung injury (ALI) and prolonged mechanical ventilation are also common. Neurological injuries, including stroke and cognitive decline, remain major concerns, particularly in older adults. ABI also increases transfusion requirements due to hemolysis and coagulopathy, which are themselves independent predictors of poor outcome (Hori et al., 2014).
Mitigation Strategies:
- Closed Circuit Designs: Closed CPB circuits eliminate open reservoirs, minimizing air exposure. Miniaturized extracorporeal circulation (MiECC) systems have demonstrated lower inflammatory marker expression and better postoperative organ function (Kofidis et al., 2008).
- Biocompatible Materials: Surface coatings such as heparin, phosphorylcholine, and nitric oxide-releasing polymers reduce protein adsorption and cellular activation on contact surfaces, thereby attenuating inflammatory and coagulative responses (Allen et al., 2005).
- Modified Suction and Venting Systems: Modern suction devices incorporate bubble traps and anti-foaming agents that reduce air entrainment. Surgical field suction should be minimized and directed to cell salvage systems where possible.
- Advanced Filtration and De-airing Techniques: Incorporating arterial line filters, venous bubble traps, and ultrasonic bubble detectors enhances the removal of air emboli. Active de-airing techniques during cardiac chamber closure and aortic unclamping are essential (Ranucci et al., 2007).
- Optimized Perfusion Practices: Reducing circuit priming volumes, using low-shear pumps, maintaining normothermia, and minimizing CPB duration can significantly reduce ABI-related complications.
Future Directions: Research into novel materials and surface coatings continues to advance. There is also growing interest in integrating sensors and real-time monitoring tools to detect and prevent ABI-related events during surgery. Artificial intelligence and machine learning may eventually optimize perfusion parameters to reduce air exposure and predict patient-specific risks.
Conclusion: The air-blood interface presents a significant pathophysiological challenge in cardiopulmonary bypass. It incites a series of deleterious responses that can significantly affect patient outcomes. Reducing ABI through technical innovation, refined surgical techniques, and adherence to perfusion best practices is critical to improving the safety and efficacy of CPB. Ongoing research is necessary to develop and validate new strategies for mitigating ABI and improving long-term patient outcomes.
References: Allen, S., McBride, W.T., Young, I.S., et al. (2005) ‘A clinical, renal and immunological assessment of surface modifying additive treated (SMART) cardiopulmonary bypass circuits’, Perfusion, 20(5), pp. 255–262.
Bellinger, D.C., Jonas, R.A., Rappaport, L.A., et al. (2001) ‘Developmental and neurological status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass’, The New England Journal of Medicine, 332(9), pp. 549–555.
Carr, B.D., Johnson, T.J., Gomez-Rexrode, A., et al. (2020) ‘Inflammatory effects of blood-air interface in a porcine cardiopulmonary bypass model’, ASAIO Journal, 66(1), pp. 72–78.
Gravlee, G.P., Davis, R.F., Kurusz, M., Utley, J.R. (2008) Cardiopulmonary Bypass: Principles and Practice. 3rd ed. Philadelphia: Lippincott Williams & Wilkins.
Hori, D., Nomura, Y., Ono, M., et al. (2014) ‘Cognitive decline after cardiac surgery: role of cytokine activation and cerebral perfusion’, Interdisciplinary Cardiovascular and Thoracic Surgery, 18(6), pp. 638–645.
Kofidis, T., Baraki, H., Singh, H., et al. (2008) ‘The minimized extracorporeal circulation system causes less inflammation and organ damage’, Perfusion, 23(3), pp. 147–151.
Paparella, D., Yau, T.M., Young, E. (2002) ‘Cardiopulmonary bypass induced inflammation: pathophysiology and treatment’, An Update on Perfusion, 17(3), pp. 213–219.
Ranucci, M., Isgro, G., Carlucci, C., et al. (2007) ‘Deairing procedures during cardiopulmonary bypass: influence on neurological outcome’, Perfusion, 22(6), pp. 407–412.
Toomasian, C.J., Aiello, S.R., Drumright, B.L., et al. (2018) ‘The effect of air exposure on leukocyte and cytokine activation in an in-vitro model of cardiotomy suction’, Perfusion, 33(7), pp. 558–564.
Asif Mushtaq: Chief Perfusionist at Punjab Institute of Cardiology, Lahore, with 27 years of experience. Passionate about ECMO, perfusion education, and advancing perfusion science internationally.
